Stress to the System: Exploring of Harmful Algal Blooms and Freshwater Acidification in the Great Lakes

Dr. Reagan Errera working with field samples.
Photo courtesy of Dr. Reagan Errera.
The Laurentian Great Lakes support an economy exceeding $82 billion in wages annually and supply drinking water to more than 40 million people. This valuable natural resource faces numerous challenges, including shifting land uses, climate induced acidification, increased aquatic temperatures, prevalent invasive species, harmful algal blooms (HABs), hypoxia, and loss of native populations. Exploring a few of these environmental stressors can provide insight into current and future scenarios that challenge the Great Lakes ecosystem while providing essential information to the scientific community, mangers, and the public.
Cyanobacterial harmful algal blooms (cHABs) occur in all five Great Lakes, with annual blooms occurring in Lake Erie, Lake Michigan (Green Bay), and Lake Huron (Saginaw Bay), in addition to the recent prevalence of blooms in Lake Superior (Reinl et al., 2020, Sterner et al., 2020). Blooms are often dominated by toxin-producing Microcystis aeruginosathat have the potential to produce a wide range of hepatotoxins, known as microcystins, and other natural products which can be detrimental to ecosystem structure and function. To inform management actions and assist with stakeholder decisions, a comprehensive understanding of the multiple interactive factors that influence M. aeruginosa growth and toxicity in the Great Lakes is needed. Understanding and interpreting the complex interactions between biological, chemical, and physical variables in coastal systems require versatile monitoring. I will discuss the our currently understanding of cHAB drivers, specifically in Lake Erie, and discuss some of the traditional and emerging technologies NOAA GLERL is employing to provided environmental data to the scientific community, managers, and public stakeholders to support decision making and enhance our understanding of blooms.
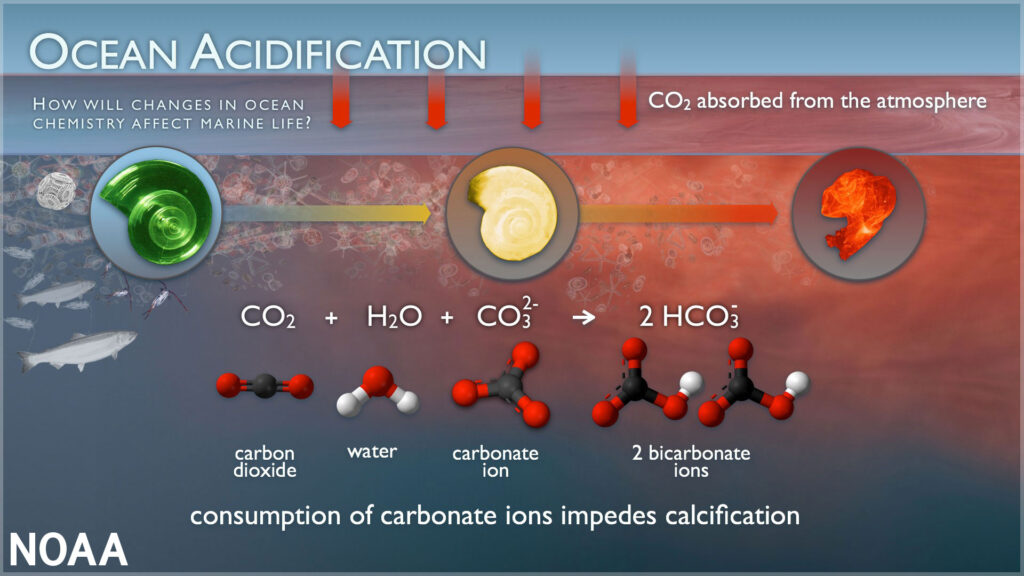
Diagram showing the impacts of Ocean Acidification on marine life.
Photo courtesy of NOAA Pacific Marine Environmental Laboratory
Climate induced ocean acidification is the result of the ocean’s uptake of increased atmospheric carbon dioxide, leading to a shift in the inorganic carbonate system and a decline in the pH. The same chemical process occurs in freshwater ecosystems but unlike oceanic systems, the Great Lakes have been widely under surveyed. Large lake systems with similar geology as Lakes Michigan, Huron, and Erie have seen a decrease in pH of 0.3 units over the past 40 years (Weiss et al., 2018), similar to the oceans. Though it is unclear if the Great Lakes have experienced a similar decrease in pH due to lack of long-term, consistent, inorganic carbonate chemistry datasets. Complicating things further, though physically interconnected, the Great Lakes encompass five distinct freshwater systems in terms of productivity (Fahnenstiel et al., 2016) and biogeochemistry (Sterner, 2021), suggesting that the impact of expected acidification will also vary between systems (Phillips et al., 2015, Sterner, 2021). In 2022, Great Lakes Environmental Research Laboratory and Thunder Bay National Marine Sanctuary teamed up to established a monitoring program for acidification in the Great Lakes. Preliminary data has been able to detect changes in the inorganic carbonate system, especially in near the unique sink hole region in Lake Huron. In addition, shifts in the coastal acidification may be occurring following storm events. This dataset is the first step in providing an understanding of the inorganic carbonate chemistry system in the Great Lakes.


